D.G. Leopold, K.K. Murray, A.E.S. Miller, W.C. Lineberger, Methylene: A study of the X̃ 3B1 and ã 1A1 states by photoelectron spectroscopy of CH2−and CD2−, J. Chem Phys. 83 (1985) 4849. doi:10.1063/1.449746.
Abstract
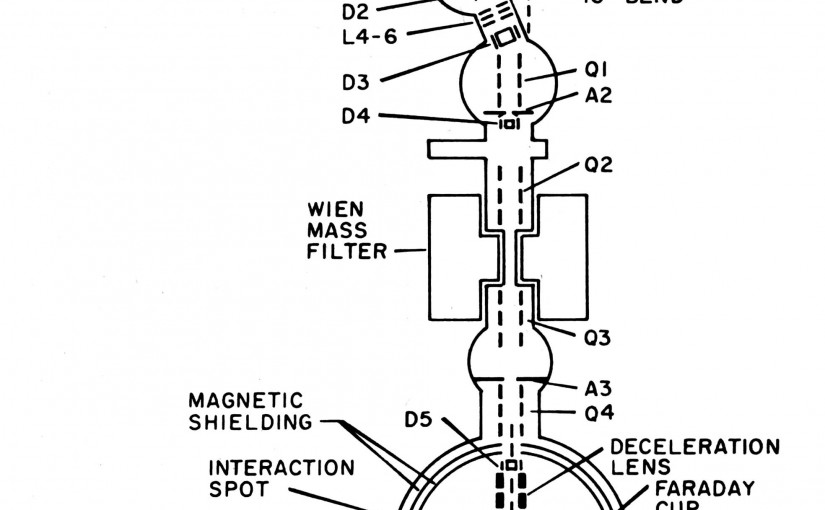
Photoelectron spectra are reported for the CH2(X̃ 3B1)+e−←CH−2 (X̃ 2B1) and CH2(ã 1A1)+e−←CH−2(X̃ 2B1) transitions of the methylene and perdeuterated methylene anions, using a new flowing afterglow photoelectron spectrometer with improved energy resolution (11 meV). Rotational relaxation of the ions to ∼300 K and partial vibrational relaxation to <1000 K in the flowing afterglow negative ion source reveal richly structured photoelectron spectra. Detailed rotational band contour analyses yield an electron affinity of 0.652±0.006 eV and a singlet–triplet splitting of 9.00±0.09 kcal/mol for CH2. (See also the following paper by Bunker and Sears.) For CD2, results give an electron affinity of 0.645±0.006 eV and a singlet–triplet splitting of 8.98±0.09 kcal/mol. Deuterium shifts suggest a zero point vibrational contribution of 0.27±0.40 kcal/mol to the observed singlet–triplet splitting, implying a Te value of 8.7±0.5 kcal/mol. Vibrational and partially resolved rotational structure is observed up to ∼9000 cm−1 above the zero point vibrational level of the 3B1 states, revealing a previously unexplored region of the quasilinear potential surface of triplet methylene. Approximately 20 new vibration‐rotation energy levels for CH2 and CD2 are measured to a precision of ∼30 cm−1 in the v2=2–7 region (bent molecule numbering). Bending vibrational frequencies in the methylene anions are determined to be 1230±30 cm−1 for CH− and 940±30 cm−1 for CD−2, and the ion equilibrium geometries are bracketed. The measured electron affinity also provides values for the bond strength and heat of formation of CH−2, and the gas phase acidity of CH3. A detailed description of the new flowing afterglow photoelectron spectrometer is given.