J.L. Laboy, K.K. Murray, “Characterization of infrared matrix-assisted laser desorption ionization samples by Fourier transform infrared attenuated total reflection spectroscopy,” Appl. Spectrosc. 58 (2004) 451–456. doi:10.1366/000370204773580301.
Abstract

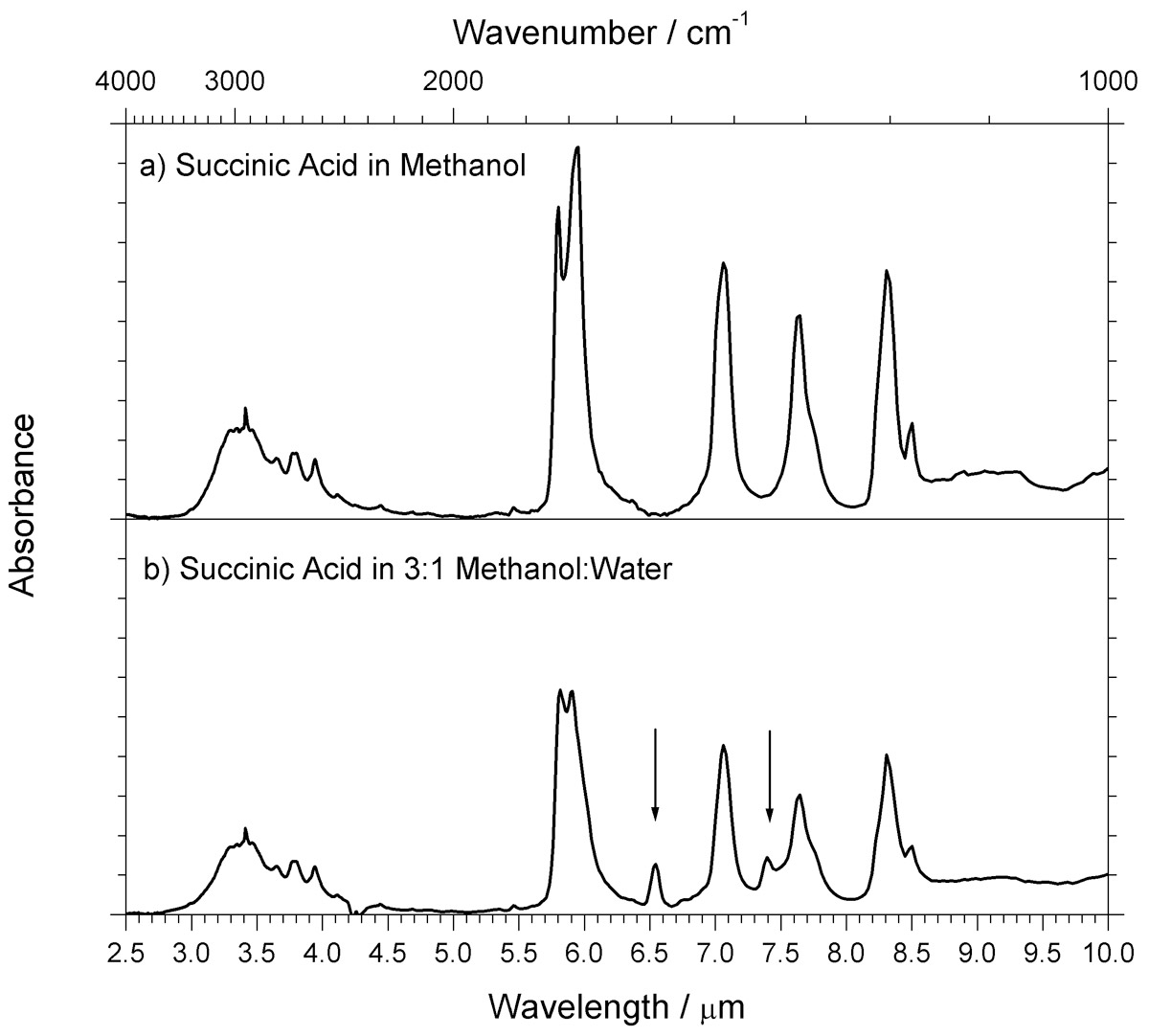
Fourier transform infrared attenuated total reflection (FT-IR ATR) spectroscopy was used to characterize thin films of succinic acid, a matrix compound commonly used with infrared matrix-assisted laser desorption ionization (IR-MALDI) mass spectrometry. IR spectra of succinic acid thin films deposited alone and in combination with the analyte biomolecules insulin and cytochrome c were obtained by FT-IR ATR spectroscopy. Spectra of analyte and matrix alone were similar to those obtained previously from KBr pellets, Nujol mull, or thin-film absorption, although the ATR spectra have significantly lower background interferences. Thin films deposited from mixtures of water and methanol have additional peaks compared to films deposited from a methanol solution. These additional peaks are attributed to carboxylate groups stabilized by residual water molecules. No evidence was found to suggest that residual water absorption contributes to absorption at wavelengths typically used for IR-MALDI. Absorption of energy by analyte vibrational modes with rapid energy transfer to the matrix is suggested as a contributor to desorption and ionization consistent with the FT-IR ATR results.