L.N.F. Darville, M.E. Merchant, V. Maccha, V.R. Siddavarapu, A. Hasan, K.K. Murray, Isolation and determination of the primary structure of a lectin protein from the serum of the American alligator (Alligator mississippiensis), Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 161 (2012) 161–169. doi:10.1016/j.cbpb.2011.11.001.
Abstract
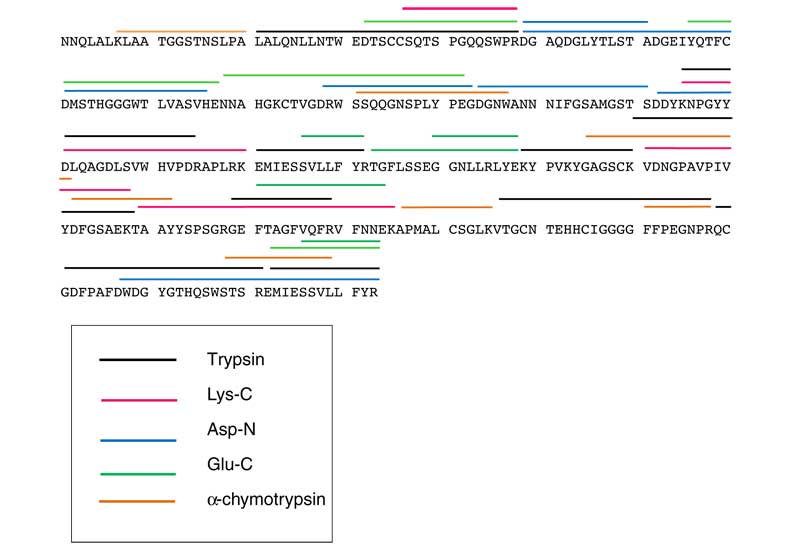
Mass spectrometry in conjunction with de novo sequencing was used to determine the amino acid sequence of a 35 kDa lectin protein isolated from the serum of the American alligator that exhibits binding to mannose. The protein N-terminal sequence was determined using Edman degradation and enzymatic digestion with different proteases was used to generate peptide fragments for analysis by liquid chromatography tandem mass spectrometry (LC MS/MS). Separate analysis of the protein digests with multiple enzymes enhanced the protein sequence coverage. De novo sequencing was accomplished using MASCOT Distiller and PEAKS software and the sequences were searched against the NCBI database using MASCOT and BLAST to identify homologous peptides. MS analysis of the intact protein indicated that it is present primarily as monomer and dimer in vitro. The isolated 35 kDa protein was ~ 98% sequenced and found to have 313 amino acids and nine cysteine residues and was identified as an alligator lectin. The alligator lectin sequence was aligned with other lectin sequences using DIALIGN and ClustalW software and was found to exhibit 58% and 59% similarity to both human and mouse intelectin-1. The alligator lectin exhibited strong binding affinities toward mannan and mannose as compared to other tested carbohydrates.

