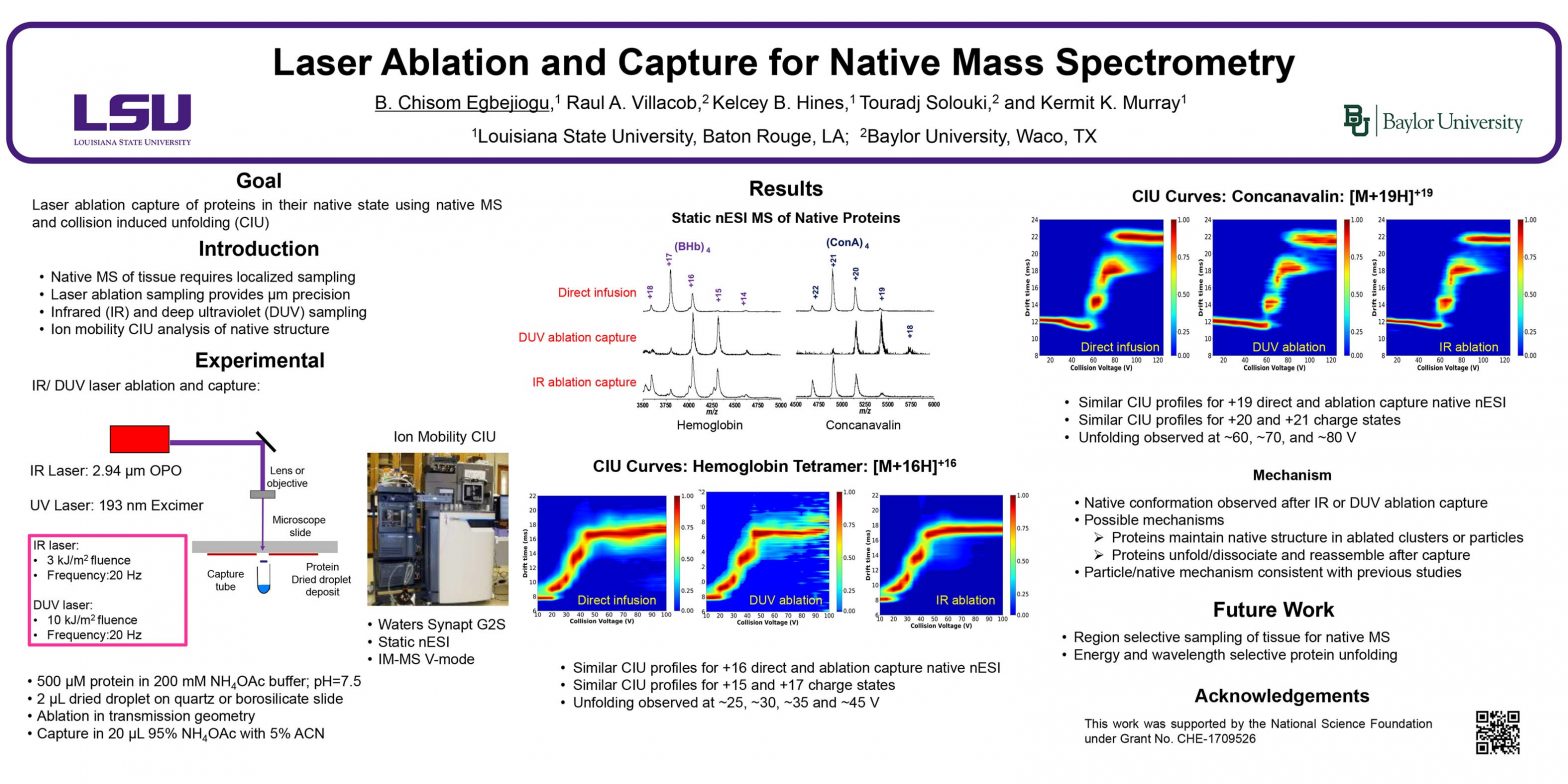
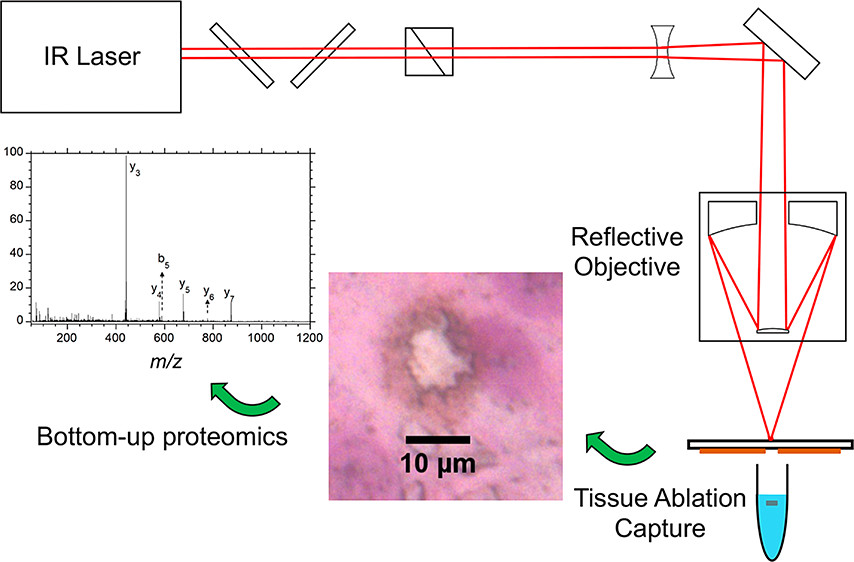
ThP 051 Premise Social media for mass spectrometry dates back to periodic newsletters sent through the mail which were followed by electronic mailing lists, Usenet newgroups, and websites, leading to the platforms of today such as Facebook and Twitter. The goal of this presentation is to quantify the use of social media use in the …
Continue reading “ASMS 2023: Social Media for Mass Spectrometry”