



Murray Mass Spectrometry Group
Research group of Kermit Murray at Louisiana State University where we use lasers for sampling and imaging and study the chemistry and physics of laser ablation.
F. Huang, K.K. Murray, “Finite element simulation of infrared laser ablation for mass spectrometry,” 26 (2012) 2145–2150. doi:10.1002/rcm.6331.
Abstract
RATIONALE: Laser ablation is widely used in conjunction with ambient ionization techniques, and a fundamental understanding of the mechanism of material removal is important to its optimal use in mass spectrometry. Finite element analysis simulates the laser material interaction on larger time and distance scales than atomistic approaches. Here, a two-dimensional finite element model was developed to simulate infrared laser irradiation of glycerol using a wavelength-tunable infrared (IR) laser.
METHODS: The laser fluence used for the simulations was varied from 1000 to 6000 J/m(2) , the wavelength was varied from 2.7 to 3.7 µm, and both flat-top and Gaussian shape laser profiles were studied.
RESULTS: Phase explosion conditions were found for laser wavelengths near 3 µm (which corresponds to the OH stretch absorption of glycerol) and fluences above 2000 J/m(2) . This suggests that laser ablation of glycerol is driven by phase explosion in the OH stretch region. The Gaussian profile generated regions of higher glycerol temperature, whereas the flat-top profile heated a larger volume of material above the phase explosion temperature.
CONCLUSIONS: These results suggest that the best performance for pulsed IR laser sample irradiation is in the wavelength range from 2.9 to 3.1 µm for materials with a strong OH stretch absorption.














S.-G. Park, K.K. Murray, “Infrared laser ablation sample transfer for MALDI imaging,” Anal. Chem. 84 (2012) 3240–3245. doi:10.1021/ac3006704.
Abstract: An infrared laser was used to ablate material from tissue sections under ambient conditions for direct collection on a matrix assisted laser desorption ionization (MALDI) target. A 10 μm thick tissue sample was placed on a microscope slide and was mounted tissue-side down between 70 and 450 μm from a second microscope slide. The two slides were mounted on a translation stage, and the tissue was scanned in two dimensions under a focused mid-infrared (IR) laser beam to transfer material to the target slide via ablation. After the material was transferred to the target slide, it was analyzed using MALDI imaging using a tandem time-of-flight mass spectrometer. Images were obtained from peptide standards for initial optimization of the system and from mouse brain tissue sections using deposition either onto a matrix precoated target or with matrix addition after sample transfer and compared with those from standard MALDI mass spectrometry imaging. The spatial resolution of the transferred material is approximately 400 μm. Laser ablation sample transfer provides several new capabilities not possible with conventional MALDI imaging including (1) ambient sampling for MALDI imaging, (2) area to spot concentration of ablated material, (3) collection of material for multiple imaging analyses, and (4) direct collection onto nanostructure assisted laser desorption ionization (NALDI) targets without blotting or ultrathin sections.



L. Darville, M.E. Merchant, K.K. Murray, A mass spectrometry approach for the study of deglycosylated proteins, “Microchem. J.,” 99 (2011) 309–311. doi:10.1016/j.microc.2011.05.020.
Abstract: Mass spectrometry (MS) analysis, after enzymatic or chemical deglycosylation, requires preparatory steps to remove salts and buffers. In this work, the glycosylated protein fetuin and a lectin protein isolated from the serum of Alligator mississippiensis were used to evaluate methods for desalting samples after an enzymatic or chemical deglycosylation. Precipitation and dialysis were used to prepare the deglycosylated samples for MS analysis. Both the precipitation and dialysis methods were suitable for sample preparation prior to analysis by matrix assisted laser desorption ionization (MALDI) MS.

L.N.F. Darville, M.E. Merchant, V. Maccha, V.R. Siddavarapu, A. Hasan, K.K. Murray, Isolation and determination of the primary structure of a lectin protein from the serum of the American alligator (Alligator mississippiensis), Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 161 (2012) 161–169. doi:10.1016/j.cbpb.2011.11.001.
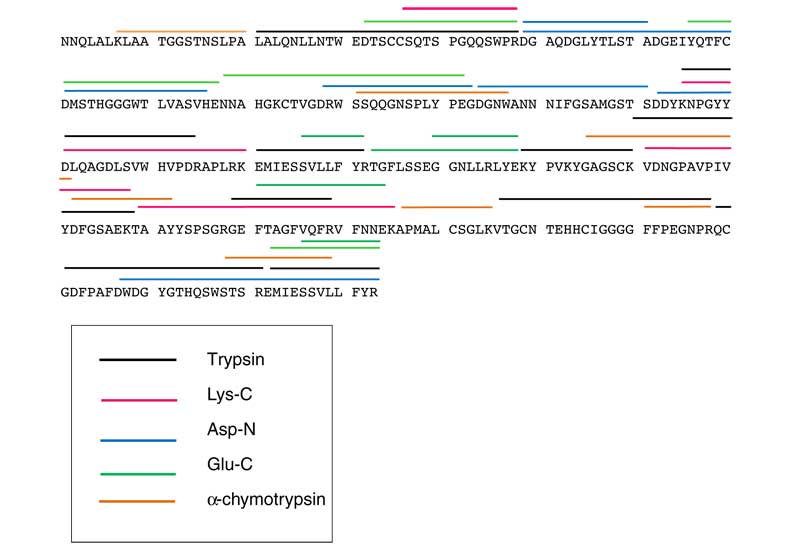
Mass spectrometry in conjunction with de novo sequencing was used to determine the amino acid sequence of a 35 kDa lectin protein isolated from the serum of the American alligator that exhibits binding to mannose. The protein N-terminal sequence was determined using Edman degradation and enzymatic digestion with different proteases was used to generate peptide fragments for analysis by liquid chromatography tandem mass spectrometry (LC MS/MS). Separate analysis of the protein digests with multiple enzymes enhanced the protein sequence coverage. De novo sequencing was accomplished using MASCOT Distiller and PEAKS software and the sequences were searched against the NCBI database using MASCOT and BLAST to identify homologous peptides. MS analysis of the intact protein indicated that it is present primarily as monomer and dimer in vitro. The isolated 35 kDa protein was ~ 98% sequenced and found to have 313 amino acids and nine cysteine residues and was identified as an alligator lectin. The alligator lectin sequence was aligned with other lectin sequences using DIALIGN and ClustalW software and was found to exhibit 58% and 59% similarity to both human and mouse intelectin-1. The alligator lectin exhibited strong binding affinities toward mannan and mannose as compared to other tested carbohydrates.
J. Lee, H. Musyimi, S. Soper, K.K. Murray, “Development of an Automated Digestion and Droplet Deposition Microfluidic Chip for MALDI-TOF MS,” J. Am. Soc. Mass Spectrom. 19 (2008) 964–972. doi:10.1016/j.jasms.2008.03.015.

An automated proteolytic digestion bioreactor and droplet deposition system was constructed with a plastic microfluidic device for off-line interfacing to matrix assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). The microfluidic chips were fabricated in poly(methyl methacrylate) (PMMA), using a micromilling machine and incorporated a bioreactor, which was 100 µm wide, 100 µm deep, and possessed a 4 cm effective channel length (400 nL volume). The chip was operated by pressure-driven flow and mounted on a robotic fraction collector system. The PMMA bioreactor contained surface immobilized trypsin, which was covalently attached to the UV-modified PMMA surface using coupling reagents N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC) and hydroxysulfosuccinimide (sulfo-NHS). The digested peptides were mixed with a MALDI matrix on-chip and deposited as discrete spots on MALDI targets. The bioreactor provided efficient digestion of a test protein, cytochrome c, at a flow rate of 1 µL/min, producing a reaction time of ~24 s to give adequate sequence coverage for protein identification. Other proteins were also evaluated using this solid-phase bioreactor. The efficiency of digestion was evaluated by monitoring the sequence coverage, which was 64%, 35%, 58%, and 47% for cytochrome c, bovine serum albumin (BSA), myoglobin, and phosphorylase b, respectively.
ASSISTANT/ASSOCIATE/FULL PROFESSOR
The Louisiana State University Department of Chemistry anticipates filling an Assistant/Associate/Full Professor (Experimental Physical Chemistry/Tenure-track) position with a starting date of August 13, 2012.
Required Qualifications: Ph.D. in chemistry or a related field; demonstrated excellence in teaching and research. Responsibilities: establish a strong, well-funded, widely recognized research program in experimental physical chemistry; teach at the undergraduate and graduate levels. An offer of employment is contingent on a satisfactory pre-employment background check.
Application deadline is November 28, 2011 or until a candidate is selected.
Applications at the Assistant Professor level should consist of a cover letter, curriculum vitae, summary of proposed research, and statement of teaching philosophy, preferably as a single PDF document; and three letters of recommendation.
Associate/Full Professor candidates should submit a letter of interest and curriculum vitae. Please submit materials electronically; see below. Arrange for letters of recommendation to be sent to Ms. Vickie Thornton (vthornton@lsu.edu), Experimental Physical Chemistry Search, Department of Chemistry, Louisiana State University, Baton Rouge, LA 70803.
LSU IS AN EQUAL OPPORTUNITY/EQUAL ACCESS EMPLOYER
Apply online at https://lsusystemcareers.lsu.edu/applicants/Central?quickFind=53755