TP 250 Murray Group ASMS 2023 Presentations
Tag Archives: MALDI
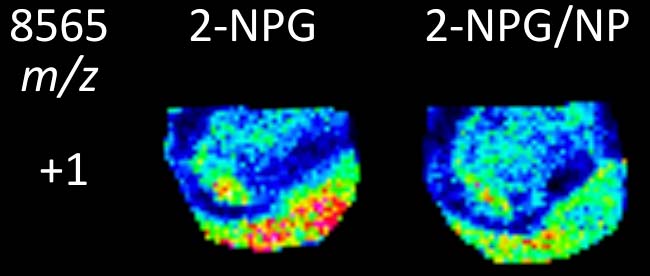
A Nanoparticle Co-matrix for Multiple Charging in MALDI Imaging of Tissue
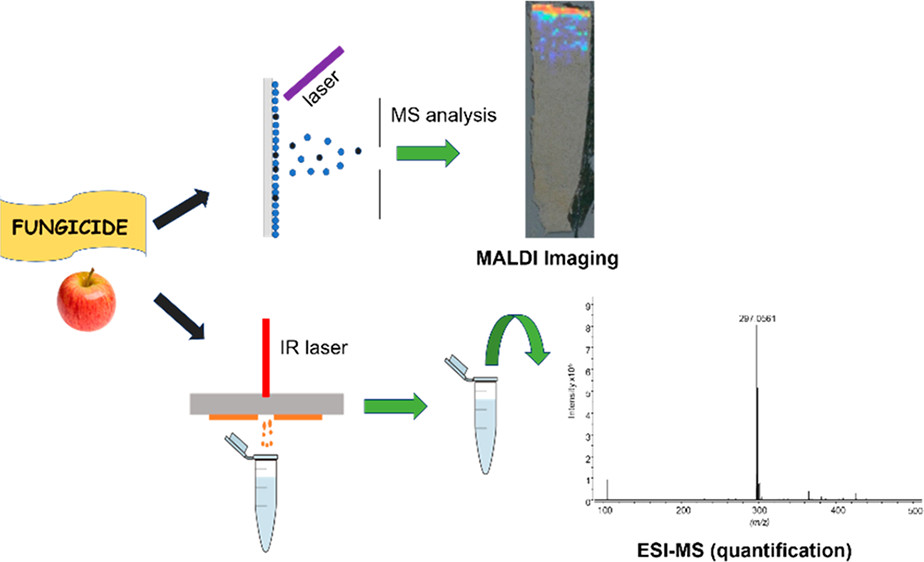
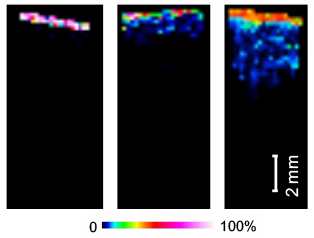
Matrix-Assisted Laser Desorption Ionization Imaging and Laser Ablation Sampling for Analysis of Fungicide Distribution in Apples
ASMS 2018: MALDI Imaging and Laser Ablation Sampling for Analysis of Fungicide Distribution in Apples
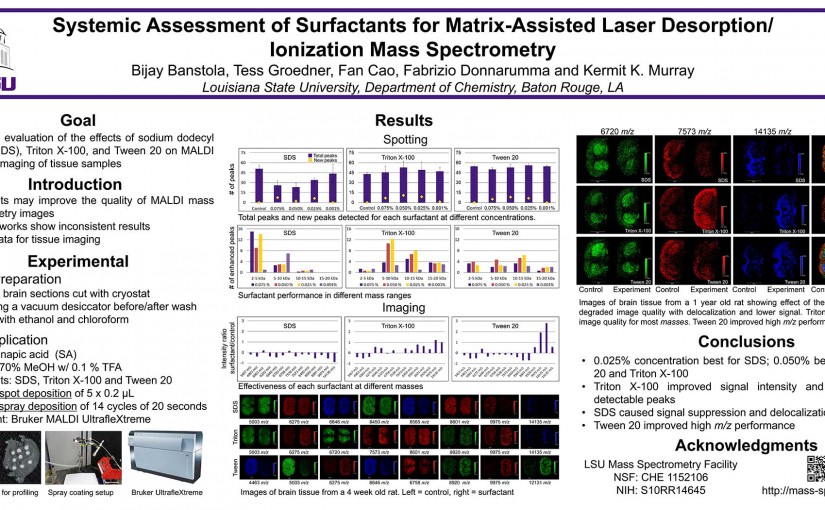
Systematic assessment of surfactants for matrix-assisted laser desorption/ionization mass spectrometry imaging
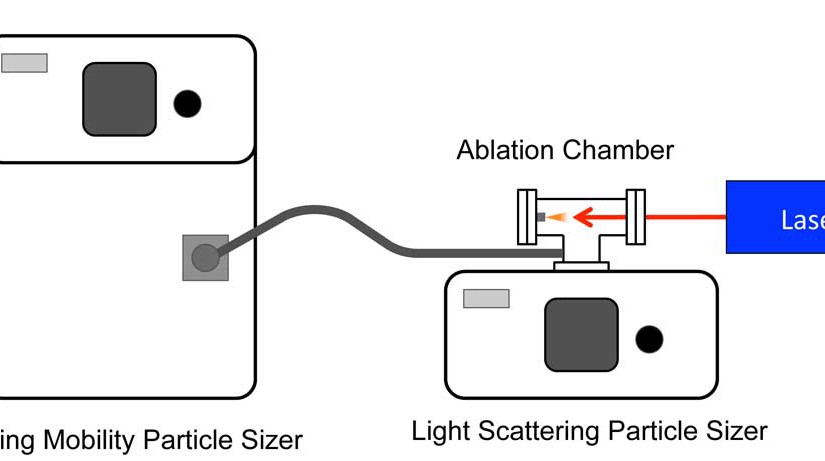
ASMS 2016: MALDI Directed Infrared Laser Ablation Sample Transfer for Spatially Resolved Biomolecule Analysis
ASMS 2016: Systematic Assessment of Surfactants for Matrix-Assisted Laser Desorption/ Ionization Mass Spectrometry
High Resolution Laser Mass Spectrometry Bioimaging
K. K. Murray, , C. A. Seneviratne, and S. Ghorai, “High Resolution Laser Mass Spectrometry Bioimaging” Methods 104 (2016) 118–126; doi:10.1016/j.ymeth.2016.03.002 Mass spectrometry imaging (MSI) was introduced more than five decades ago with secondary ion mass spectrometry (SIMS) and a decade later with laser desorption/ionization (LDI) mass spectrometry (MS). Large biomolecule imaging by matrix-assisted laser …
Continue reading “High Resolution Laser Mass Spectrometry Bioimaging”
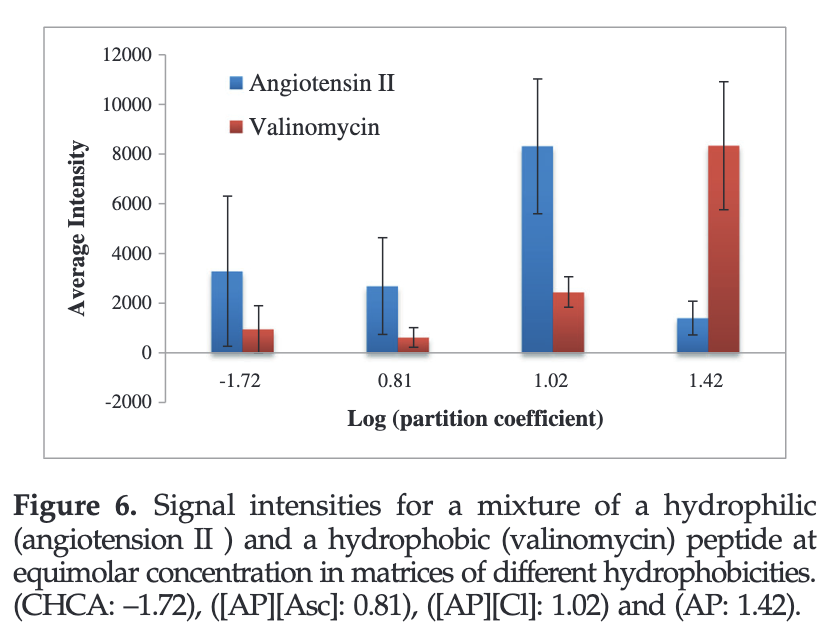
GUMBOS matrices of variable hydrophobicity for matrix-assisted laser desorption/ionization mass spectrometry
Al Ghafly, Siraj, Das, Regmi, Magut, Galpothdeniya, Murray, Warner, Rapid Commun. Mass Spectrom. 2014, 28, 2307; DOI: 10.1002/rcm.7027. RATIONALE Detection of hydrophobic peptides remains a major obstacle for matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS). This stems from the fact that most matrices for MALDI are hydrophilic and therefore have low affinities for hydrophobic peptides. Herein, …