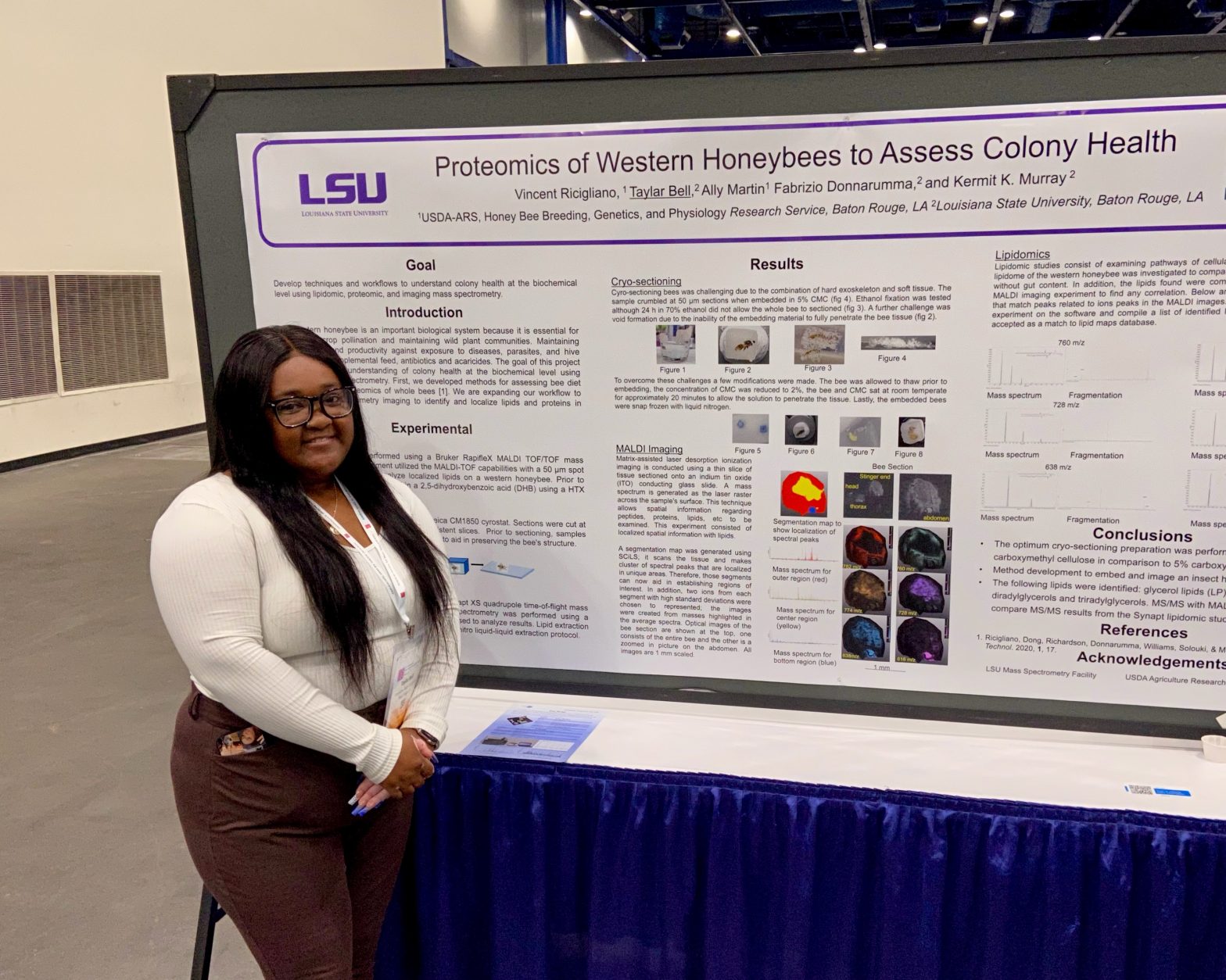
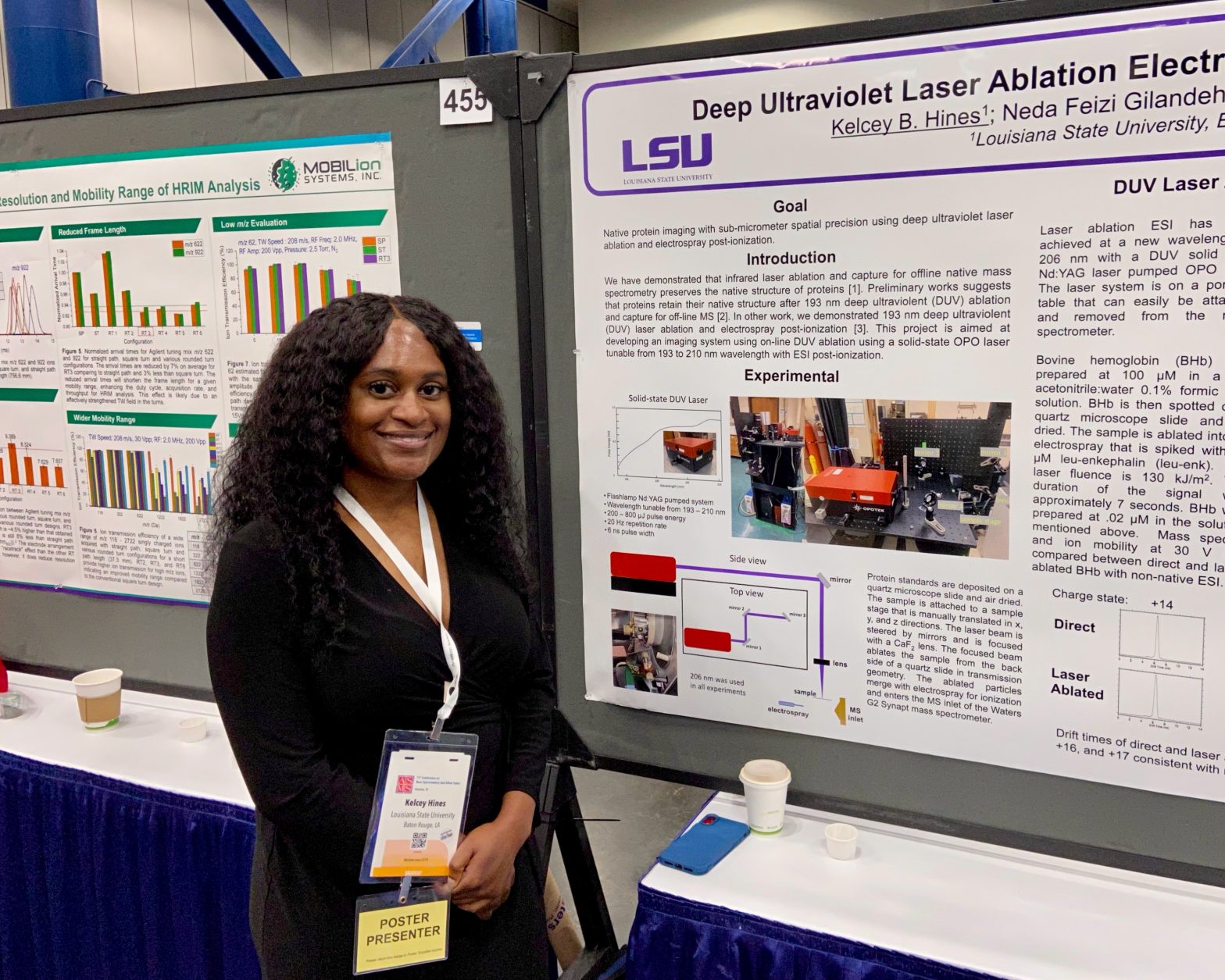
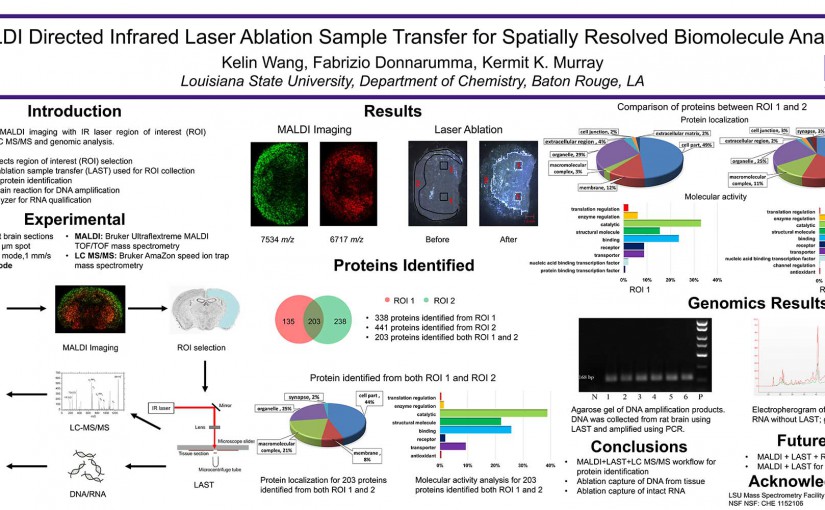
TP 250 Murray Group ASMS 2023 Presentations
Tag Archives: Proteomics
ASMS 2023: Deep Ultraviolet Laser Ablation Electrospray Ionization for Native Mass Spectrometry
WP 456 Murray Group ASMS 2023 Presentations
ASMS 2016: MALDI Directed Infrared Laser Ablation Sample Transfer for Spatially Resolved Biomolecule Analysis
Laser Ablation Sample Transfer for Localized LC-MS/MS Proteomic Analysis of Tissue
Single Cell chemical Imaging via Nanoscale IR Ablation Mass Spectromety
STTR Grant Award, Kermit Murray, PI: Nanoscale Laser Ablation Capture Mass Spectrometry for Single Cell Proteomics, National Institutes of Health, R41 GM106454
A mass spectrometry approach for the study of deglycosylated proteins
Isolation and determination of the primary structure of a lectin protein from the serum of the american alligator (Alligator Mississippiensis)
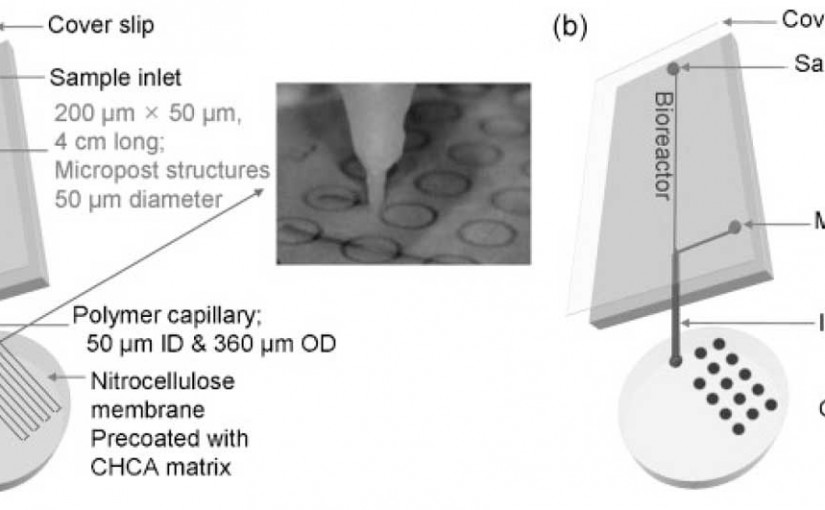
A solid-phase bioreactor with continuous sample deposition for matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
Proteome analysis of the leukocytes from the American alligator (Alligator mississippiensis) using mass spectrometry
Microfluidics with MALDI analysis for proteomics
J. Lee, S.A. Soper, K.K. Murray, “Microfluidics with MALDI analysis for proteomics–a review,” Anal. Chim. Acta. 649 (2009) 180–190. doi:10.1016/j.aca.2009.07.037. Abstract Various microfluidic devices have been developed for proteomic analyses and many of these have been designed specifically for mass spectrometry detection. In this review, we present an overview of chip fabrication, microfluidic components, and …
Continue reading “Microfluidics with MALDI analysis for proteomics”