F. Huang, X. Fan, K.K. Murray, Matrix-assisted laser desorption ionization of infrared laser ablated particles, Int. J. Mass Spectrom. 274 (2008) 21–24. doi:10.1016/j.ijms.2008.04.006.
Abstract

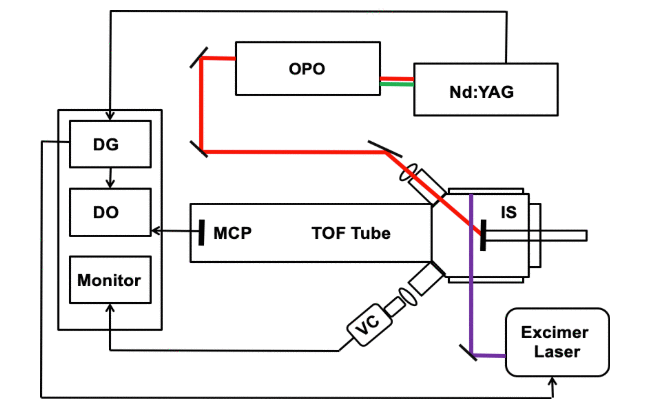
An infrared (IR) laser was used to ablate particles that were subsequently ionized by matrix-assisted laser desorption ionization (MALDI). Infrared light from a pulsed optical parametric oscillator (OPO) laser system was directed at a solid sample under vacuum containing a 2,5-dihydroxybenzoic acid (DHB) matrix and peptide or protein analyte. A pulsed 351 nm ultraviolet (UV) excimer laser that was directed 1.4 mm above and parallel to the sample surface was used to irradiate the ablated material in the desorption plume. Ions created by post-ablation ionization were detected with a linear time-of-flight (TOF) mass spectrometer. Mass spectra of the peptide bradykinin and proteins bovine insulin and cytochrome c were recorded. Under these conditions, two simultaneous mass spectra were generated: an IR–MALDI mass spectrum from the OPO and a UV post-ablation spectrum generated by irradiating material in the plume. Factors affecting the two-laser ion yield were studied, including the delay time between the laser pulses and the fluence of the IR and UV laser.

“Two possibilities for ionization of biomolecules in the plume of desorbed and ablated material are the multiphoton ionization of free molecules and the ionization of particles containing matrix and analyte. The observation of biomolecules of the size of insulin and cytochrome c (Fig. 5) argues strongly against a multiphoton ionization mechanism. Molecules of this size are difficult to ionize through the absorption of multiple photons due to the efficient energy dissipation in the large number of vibrational degrees of freedom [10]. Instead, these results suggest that particles containing a UV MALDI matrix and analyte, when irradiated with the UV laser, form ions by a MALDI process. It has been observed that particles containing matrix and analyte, when sprayed into vacuum and irradiated with a UV laser, form ions by MALDI [11,17]. A particle MALDI mechanism has been suggested previously for IR laser ablated particles that were irradiated with a second IR laser. However, in this case, the absorption of the second IR laser energy by the analyte molecule [19] or waters of hydration [20] cannot be ruled out. The observation of ions from proteins by UV irradiation of the ablated material strongly suggests that the ionization mechanism is MALDI of the ablated particles. This hypothesis is supported by results of time-resolved fast-flash photography of the glycerol plume [21] and by measurements of the particle size and number ablated by an IR laser from a MALDI matrix [14],which shows a large number of particles in the IR ablation plume.”