Author Archives: kkmurray
Infrared laser ablation and capture of enzymes with conserved activity
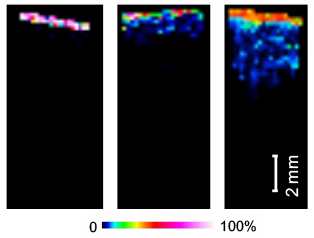
Wang, K., Donnarumma, F., Baldone, M. D., & Murray, K. K. Infrared laser ablation and capture of enzymes with conserved activity. Anal Chim Acta, 1027, 41–46 (2018). Abstract Infrared (IR) laser ablation at 3 μm wavelength was used to extract enzymes from tissue and quantitatively determine their activity. Experiments were conducted with trypsin, which was ablated, …
Continue reading “Infrared laser ablation and capture of enzymes with conserved activity”
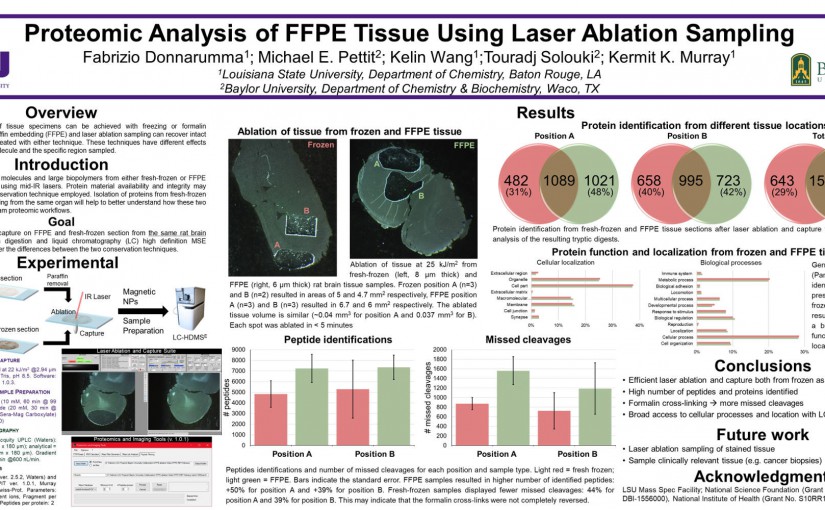
ASMS 2018: Proteomic Analysis of FFPE Tissue Using Laser Ablation Sampling
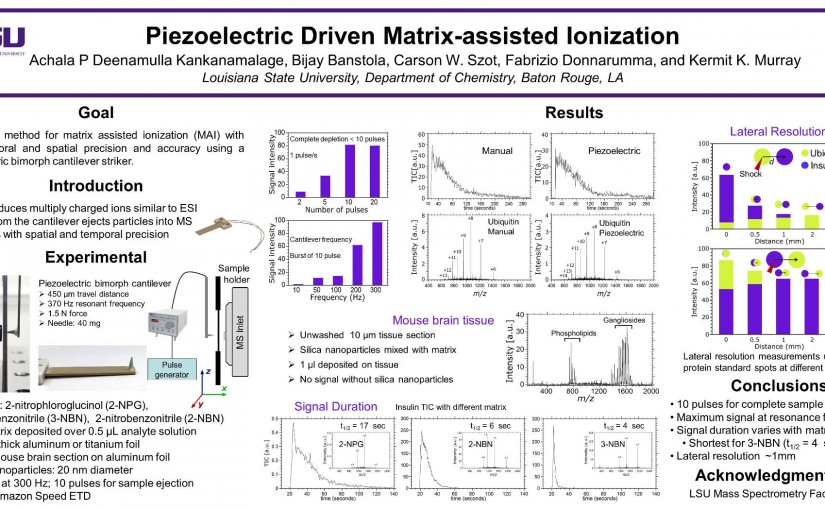
ASMS 2018: Piezoelectric Driven Matrix-assisted Ionization
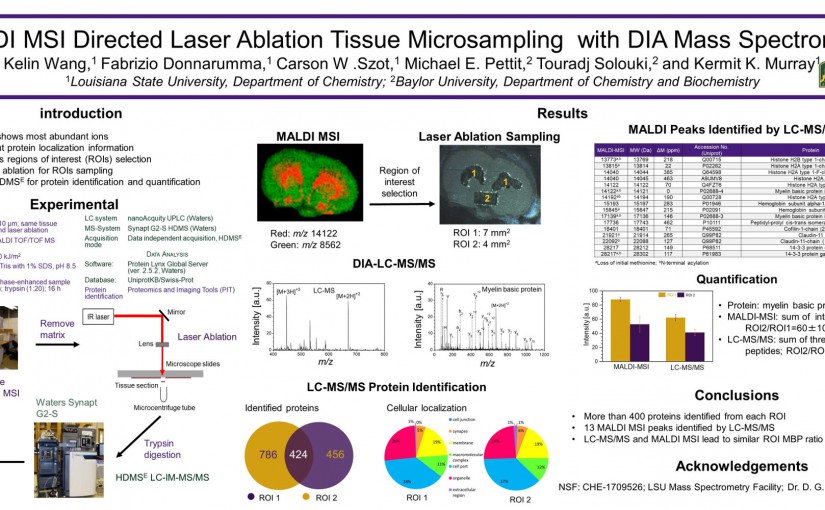
ASMS 2018: MALDI MSI Directed Laser Ablation Tissue Microsampling with DIA Mass Spectrometry
ASMS 2018: Droplet Capture Tip-enhanced Laser Ablation Sampling for Mass Spectrometry
Contact: More Information:
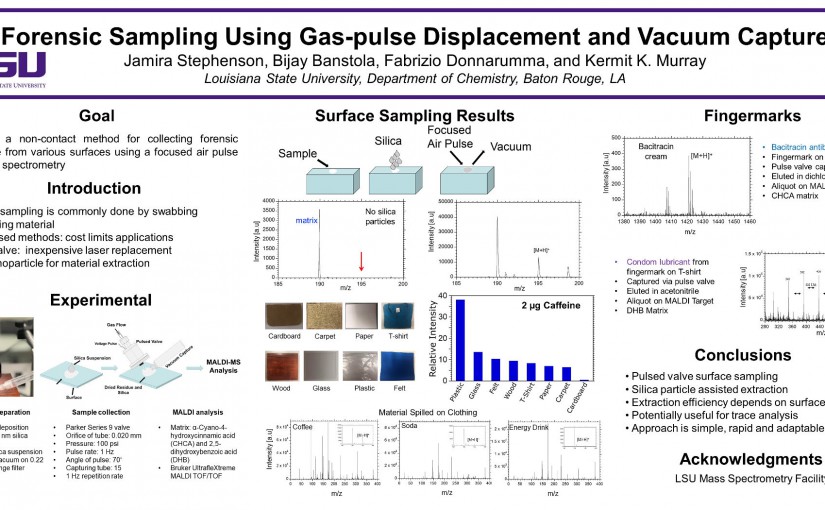
ASMS 2018: Forensic Sampling Using Gas-pulse Displacement and Vacuum Capture
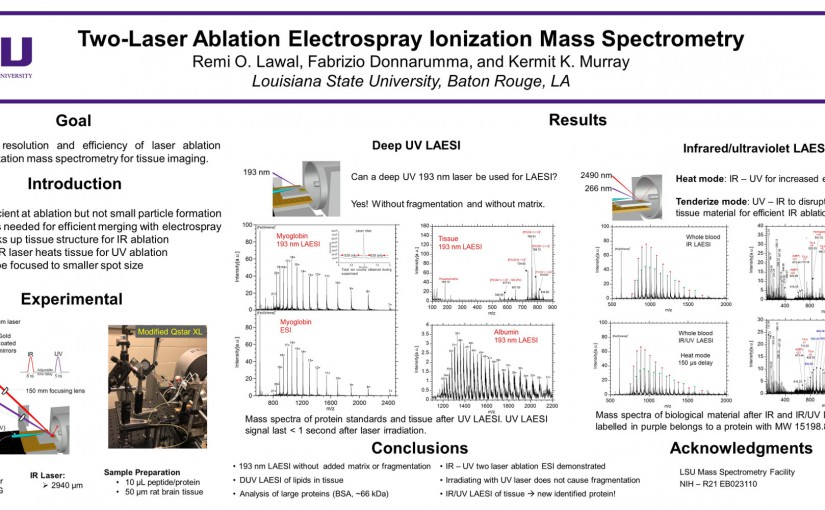
ASMS 2018: Two-laser ablation electrospray ionization mass spectrometry
ASMS 2018: MALDI Imaging of Multiply Charged Ions from Tissue using a Nanoparticle Co-matrix
Contact:
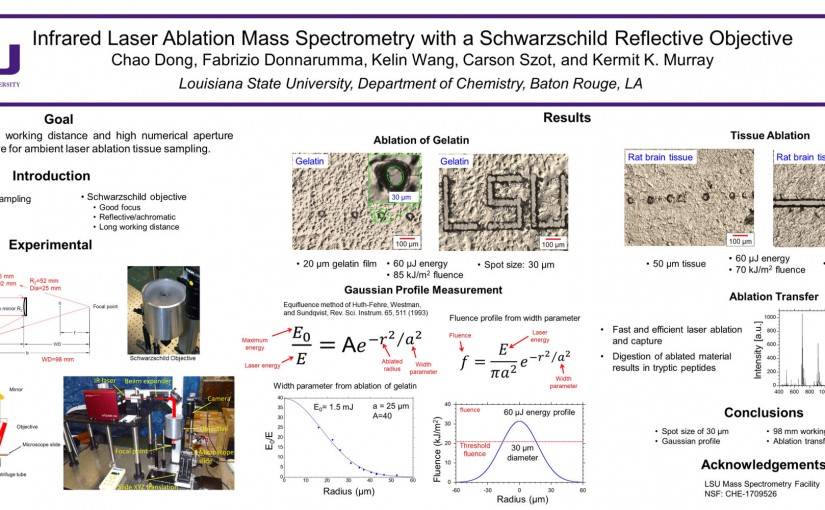
ASMS 2018: IR Laser Ablation MS with a Schwarzschild Reflective Objective
Contact: More Information: