MP252 AuthorsNeda Feizi Gilandeh1; Blessing Chisom Egbejiogu2; Kyle L. Wilhelm1; Kermit K. Murray2; Touradj Solouki1 Institutes1Baylor University, Waco, TX; 2Louisiana State University, Baton Rouge, LA IntroductionNative electrospray ionization mass spectrometry (n-ESI/MS) combined with ion mobility spectrometry (IMS) provides an opportunity to study indigenous forms of proteins in biological samples. Recently, we reported on combining n-ESI/MS …
Tag Archives: Infrared
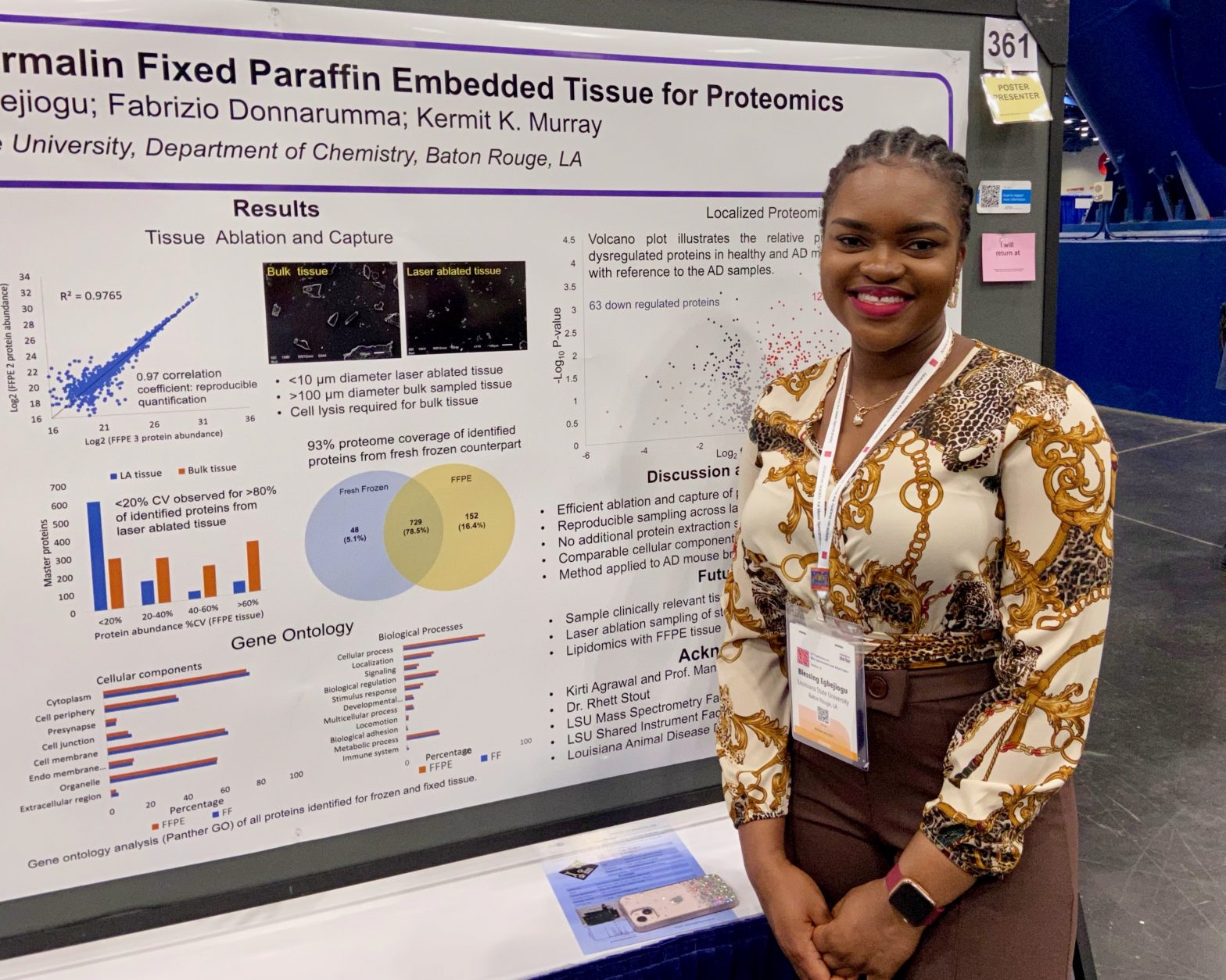
ASMS 2023: Localized Microsampling of Formalin Fixed Paraffin Embedded Tissue for Proteomics
TP361 Murray Group ASMS 2023 Presentations
Opotek Customer Spotlight
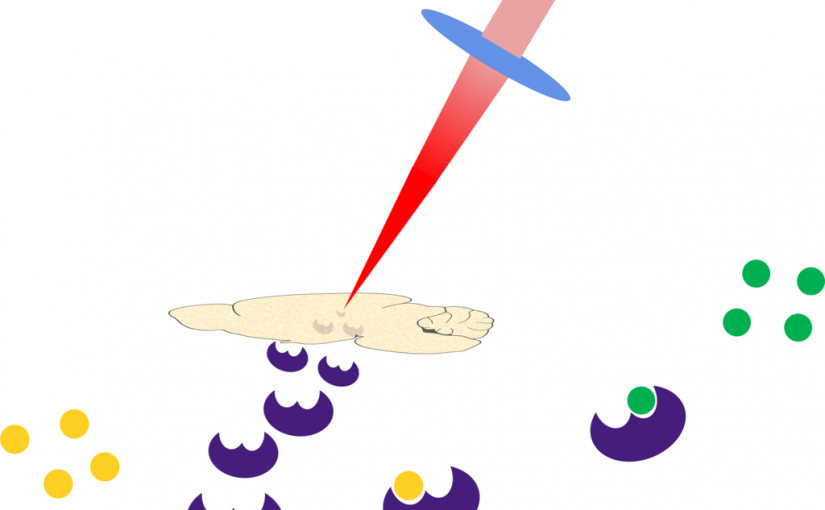
ASMS 2019: Cellular Precision for Infrared Laser Ablation Tissue Microproteomics
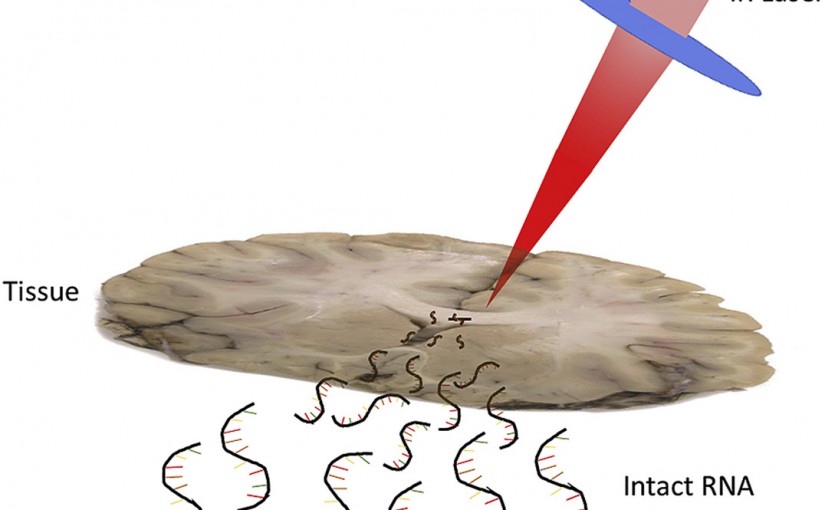
RNA Sampling from Tissue Sections using Infrared Laser Ablation
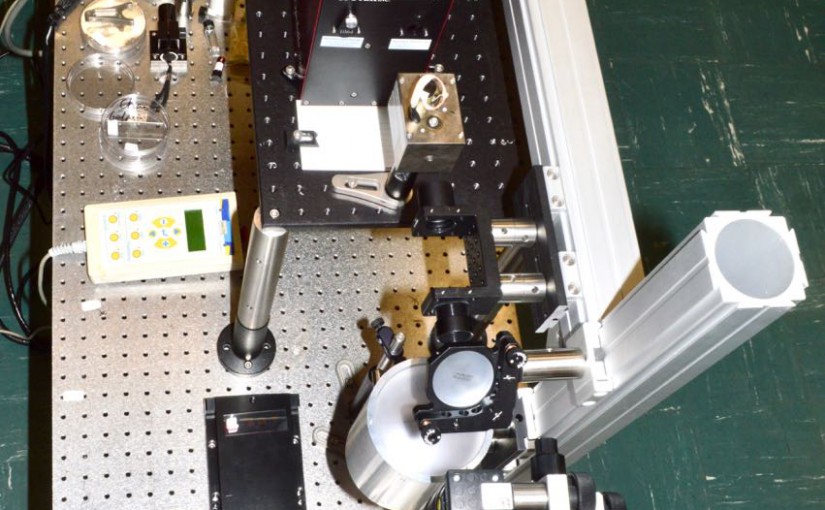
IMSC 2018: Infrared Laser Ablation Microsampling Coupled with MALDI Imaging
International Mass Spectrometry Conference, Florence, Italy, August 28, 2018 Louisiana State University: Kermit K. Murray, Fabrizio Donnarumma, Kelin Wang, Carson W. Szot, Chao Dong,Baylor University: Touradj Solouki, Michael E. Pettit Introduction Matrix-assisted laser desorption/ionization (MALDI) mass spectrometry imaging (MSI) is a powerful method for determining the location of biomolecules in tissue; however, protein identification and …
Continue reading “IMSC 2018: Infrared Laser Ablation Microsampling Coupled with MALDI Imaging”
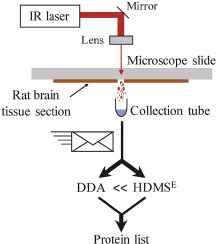
Infrared laser ablation sampling coupled with data independent high resolution UPLC-IM-MS/MS for tissue analysis
Infrared laser ablation and capture of enzymes with conserved activity
Wang, K., Donnarumma, F., Baldone, M. D., & Murray, K. K. Infrared laser ablation and capture of enzymes with conserved activity. Anal Chim Acta, 1027, 41–46 (2018). Abstract Infrared (IR) laser ablation at 3 μm wavelength was used to extract enzymes from tissue and quantitatively determine their activity. Experiments were conducted with trypsin, which was ablated, …
Continue reading “Infrared laser ablation and capture of enzymes with conserved activity”
2018 Spring ACS: Development of an Infrared Laser Ablation Microsampling System
IR Opolette 2940
Another laser and the second from OPOTEK this year: an IR Opolette 2940. It is fixed wavelength at 2940 nm for no other reason than that is the Er:YAG laser wavelength. It is possible to manually adjust the internal optics to generate light from 2700 to 3100 nm. This laser has its roots in the …